Interest in drug repurposing for cancer research continues to grow. One compound drawing attention is Fenbendazole (FBZ), a medication long used in veterinary care. A peer-reviewed study by Australian researchers explored how advanced nanoparticle delivery systems may improve Fenbendazole absorption and anticancer activity. In a published study , Esfahani et al. (2022) demonstrated that nanotechnology can significantly enhance how this compound interacts with prostate cancer cells.
The Challenge of Fenbendazole Bioavailability
Although preclinical studies highlight Fenbendazole’s anticancer potential, poor water solubility limits its effectiveness. Low solubility reduces bioavailability, which means less of the compound reaches target cells. This challenge affects many therapeutic compounds and often requires formulation innovation to overcome.
Nanoparticle-Based Delivery Approach
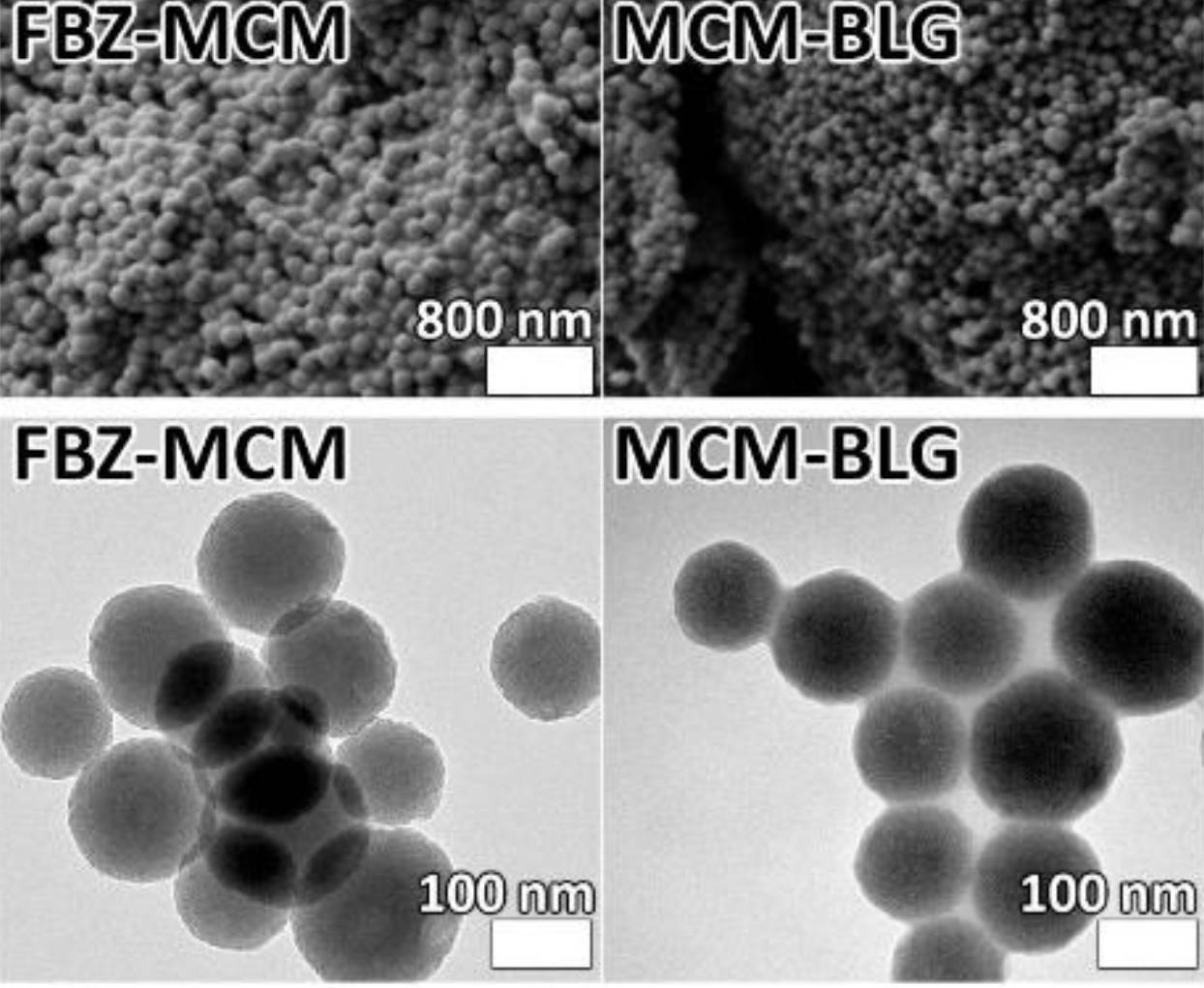
To address this limitation, the research team developed a novel nano-delivery system. They used mesoporous silica nanoparticles (MCM-48) and functionalized them with succinylated β-lactoglobulin (BLG). This coating helped control drug release and improved stability during delivery.
The resulting formulation, referred to as FBZ-MCM-BLG, significantly increased water solubility. When tested on human prostate cancer (PC-3) cells, the nanoformulation showed enhanced biological activity compared to non-encapsulated Fenbendazole.
Observed Effects in Prostate Cancer Cells
The study reported notable improvements in cytotoxic activity. FBZ-MCM-BLG demonstrated up to 5.6-fold higher cytotoxicity than free Fenbendazole and 1.8-fold higher activity than earlier nanoparticle formulations. In addition, researchers observed a 1.6-fold increase in reactive oxygen species (ROS), which play a role in cancer cell death.
Importantly, the nanoparticle formulation also reduced cancer cell migration. Because metastasis remains a leading cause of cancer mortality, this finding suggests that improved delivery systems may influence both tumor growth and spread at the cellular level.
What This Means for Future Cancer Research
These findings mark a meaningful step in Fenbendazole research. However, questions remain. Researchers must evaluate this nano-delivery system in animal models and clinical settings to confirm safety, effectiveness, and scalability.
Nanoparticle formulations can introduce additional considerations, including toxicity and long-term tolerability. For this reason, further investigation remains essential before any clinical application becomes possible.
Growing Pharmaceutical Interest
Notably, pharmaceutical researchers have increased their focus on Fenbendazole-based formulations. While the compound originated as a veterinary medication, its cellular activity has sparked interest in advanced drug-delivery research.
This attention reflects a broader trend in oncology: improving known compounds through formulation science. In this case, nanoparticles appear to enhance delivery without altering the compound’s core biological properties.
Key Takeaways from the Study
The Australian study highlights how nanotechnology may improve Fenbendazole absorption and cellular interaction. While nanoparticle systems can optimize delivery, the compound’s inherent activity remains central to observed effects.
As research continues, formulation science may play an important role in determining how such compounds evolve within oncology research frameworks.
For full experimental details, refer to the original publication .
Ongoing research will determine how nanoparticle delivery systems shape the future of Fenbendazole-related cancer studies.