Thyroid cancer represents the most common endocrine malignancy worldwide. Among its subtypes, papillary thyroid cancer (PTC) accounts for roughly 80% of diagnosed cases. Although most patients experience favorable outcomes, a subset develops persistent or progressive disease. In these cases, tumors may dedifferentiate into anaplastic thyroid cancer (ATC), an aggressive form associated with rapid progression and poor survival. Because standard therapies often fail in advanced disease, researchers continue to explore safer and more effective treatment strategies.
The Role of Mebendazole in Thyroid Cancer Research
Recent preclinical research has examined mebendazole, a benzimidazole compound traditionally used as an antiparasitic medication, for its potential activity against thyroid cancer. A study conducted at Johns Hopkins University evaluated whether this well-known drug could suppress tumor growth and prevent metastasis, particularly during early disease stages before widespread progression occurs.
In Vitro Activity Against Thyroid Cancer Cells
Laboratory experiments demonstrated that mebendazole inhibited growth across several human thyroid cancer cell lines. The compound reduced viability in both papillary (B-CPAP) and anaplastic (8505c) thyroid cancer cells. Importantly, researchers observed strong cytotoxic effects at low micromolar concentrations.
In aggressive anaplastic thyroid cancer cells, mebendazole also reduced migratory and invasive behavior. Because these traits drive rapid disease spread, limiting them represents an important therapeutic goal in advanced thyroid cancer research.
In Vivo Findings in Thyroid Cancer Models
To further evaluate therapeutic potential, researchers tested mebendazole in orthotopic thyroid cancer models. Treatment led to significant regression of papillary thyroid tumors and slowed the growth of anaplastic tumors. Tumors from treated animals showed lower expression of the proliferation marker Ki-67, indicating reduced cell division.
In addition, mebendazole decreased tumor vascularity. Researchers observed reduced vascular endothelial growth factor (VEGF) expression, suggesting impaired blood vessel formation. Together, these effects point to a dual mechanism that limits tumor expansion and nutrient supply.
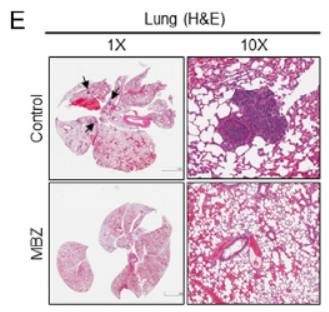
Most notably, daily oral administration prevented established thyroid tumors from spreading to the lungs. Lung metastasis commonly occurs in anaplastic thyroid cancer and contributes to poor outcomes. In contrast, untreated control animals developed extensive pulmonary metastases.
Clinical Relevance and At-Risk Patient Groups
These findings hold relevance for the estimated 20–30% of papillary thyroid cancer patients who experience persistent or progressive disease. This group faces a higher risk of dedifferentiation and reduced survival, particularly once standard treatments lose effectiveness.
Mebendazole’s long-standing safety record strengthens its appeal for further investigation. The drug has been widely used in pediatric and adult populations with minimal toxicity. This profile suggests potential use as an adjunct therapy alongside surgery, radiotherapy, or targeted treatments, pending clinical validation.
Importantly, patients with radioiodine-refractory disease often have limited options. For this reason, researchers continue to explore whether safe, low-toxicity compounds like mebendazole could improve long-term disease control.
Related Compounds: Fenbendazole
Within the same benzimidazole family, fenbendazole has attracted attention for its possible anticancer activity in preclinical settings. Reports describe activity in several cancer models, although controlled clinical data remain limited. As a result, fenbendazole research continues largely outside formal oncology frameworks.
By comparison, mebendazole benefits from extensive clinical use and regulatory familiarity. This background supports its further evaluation in oncology-focused research, particularly for aggressive thyroid cancers.
Conclusion
Preclinical evidence suggests that mebendazole may suppress tumor growth and reduce metastasis in thyroid cancer models, including highly aggressive anaplastic disease. Its favorable safety profile and demonstrated biological activity make it a strong candidate for further investigation.
Although additional studies and clinical trials are necessary, this research highlights the broader potential of drug repurposing in oncology. As scientists seek more effective strategies for advanced thyroid cancer, mebendazole may represent a promising avenue for future therapeutic development.